Law sanctioned by President Luiz Inácio Lula da Silva and published this Friday (15) in Official Journal of the Union defines the rules for human vaccination in private establishments. The text provides that sites must be approved for the activity by a competent health authority and that they must have a technical manager with medical, pharmaceutical or nursing training.
“The vaccination service will have a legally qualified professional to carry out vaccination activities during the entire period in which the service is offered,” underlines the publication. “Professionals involved in vaccination processes will be periodically trained for the service, in accordance with regulations.”
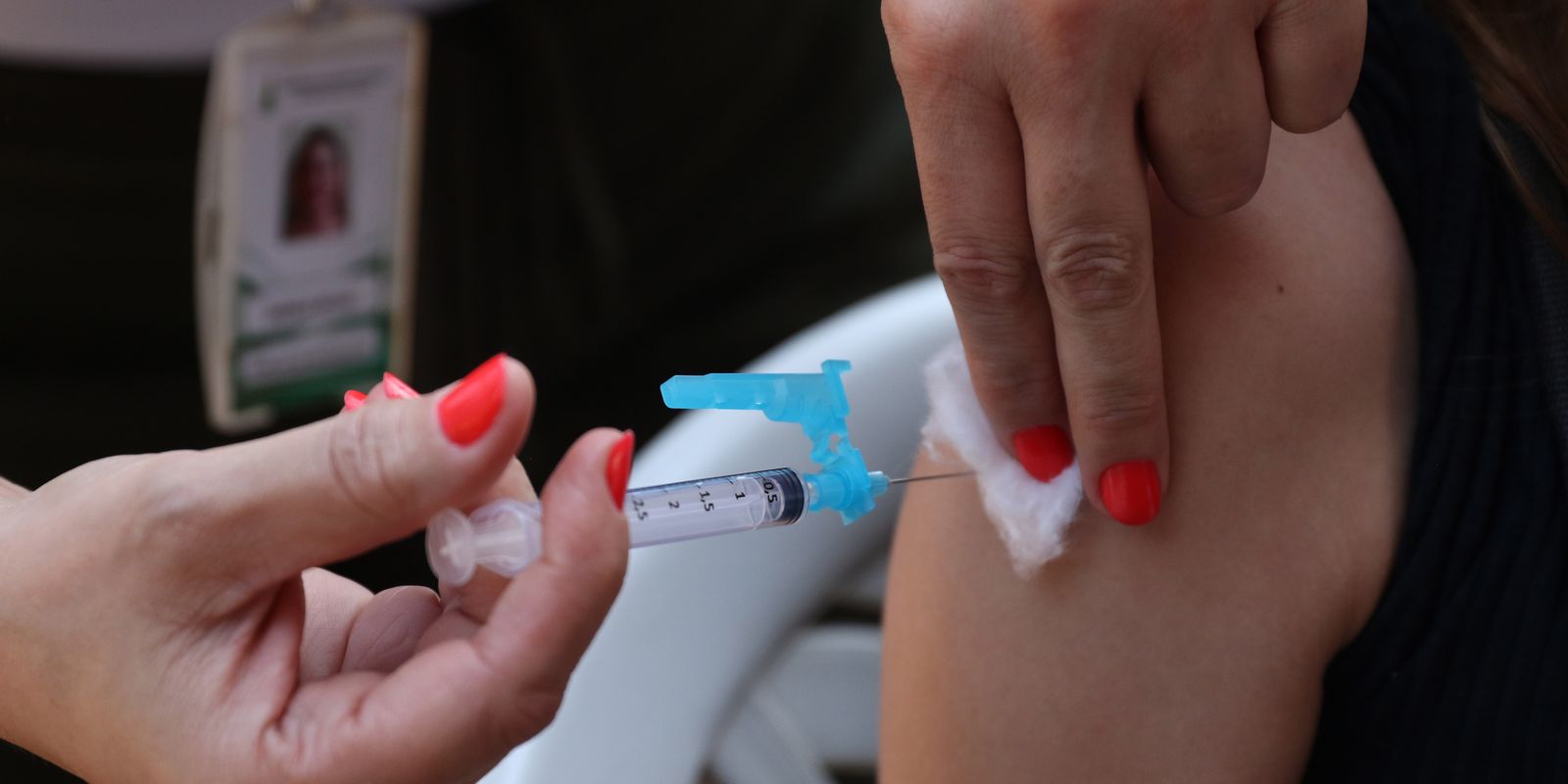
Still according to the text, vaccination services must manage technologies, processes and procedures, in accordance with applicable health standards, to preserve the safety and health of the user, and adopt procedures to maintain quality and integrity vaccines in the cold network, including during transport;
Furthermore, the places concerned must record the following information on the vaccination certificate, in a legible manner, and in the information systems defined by the managers of the Unified Health System (SUS): identification of the establishment; identification of the vaccinated person and the vaccinator; vaccine data: name, manufacturer, batch number and dose; date of vaccination; and the date of the next dose, if applicable.
The services must also keep an individual medical file listing all vaccines administered, accessible to the user and the health authority, in compliance with confidentiality rules; keep documents proving the origin of the vaccines used available to the health authority; report the occurrence of post-vaccination adverse events, including vaccination errors.
The law cites the right of users of vaccination services to control the removal of the material to be applied from its place of refrigeration or storage; check the name and validity of the product that will be applied; receive information regarding contraindications; receive advice on what to do in the event of post-vaccination adverse events; be informed of all procedures carried out during vaccination.
“Failure to comply with the provisions contained in this law constitutes a health offense under the terms of Law No. 6.437 of August 20, 1977, without prejudice to applicable civil, administrative and criminal responsibilities,” the publication specifies. The text comes into force in 90 days.

“Typical thinker. Unapologetic alcoholaholic. Internet fanatic. Pop culture advocate. Tv junkie.”


:strip_icc()/i.s3.glbimg.com/v1/AUTH_da025474c0c44edd99332dddb09cabe8/internal_photos/bs/2023/D/n/i7eIkcQsAB0YgsWKDQMw/whatsapp-image-2023-03-08-at-10.48.49.jpeg)




:strip_icc()/i.s3.glbimg.com/v1/AUTH_63b422c2caee4269b8b34177e8876b93/internal_photos/bs/2021/T/V/IFkavRRSSjHCBPZfqVXw/ap21313490536644.jpg)